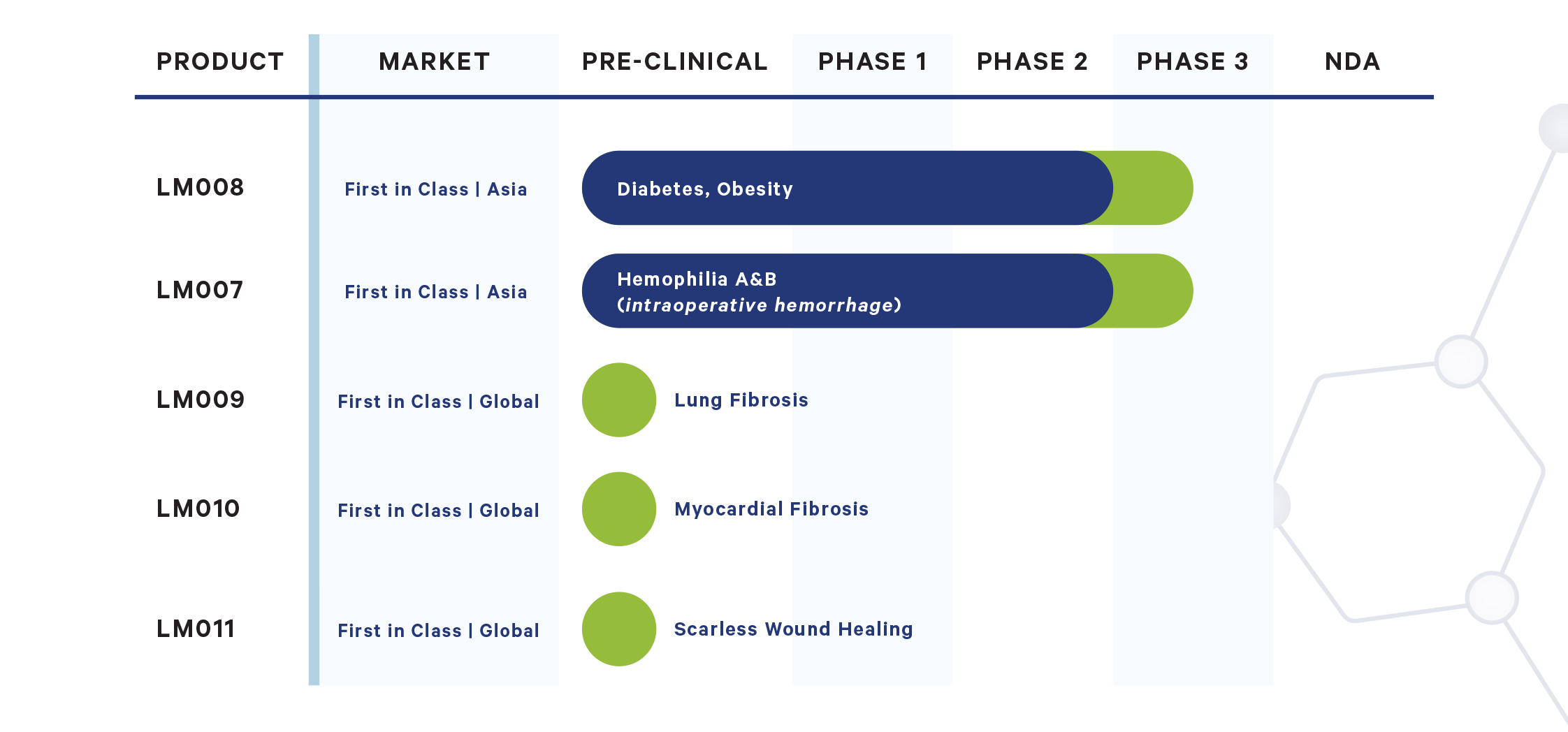
Pipeline
Our
Main
Pipeline
LeaderMed Pharmaceuticals is developing first-in-class drug pipeline to address a significant deficit in medical treatment needs. Our pharmaceutical development operations have engaged separately top-rated Clinical Research Organization (CRO) and Contract Development and Manufacturing Organization (CDMO) providing maximum IP protection for its collaborators.
Market
- Approximately 227 million people live with Type-2 diabetes in Asia, 62% of diabetes patients in the world live in Asia.
- China has the highest number of diabetics worldwide, with approximately 141 million people suffering from the disease.
- By year 2045, it is predicted that China will have around 174 million people with diabetes.
- Studies have shown that Asians are at higher risk of developing Type-2 diabetes at the same BMI.
Development Plan
- Streamlined large-scale manufacturing ongoing in China
- IND preparation ongoing
- Autoinjectors being tested for selection and development
- Preparation for Phase III in obesity and diabetes in China ongoing
- LM008 is targeting initiation of Phase III trials for diabetes and obesity by end of 1Q 2023, and approval in China by 2026.
LM 008
- First-in-Class world’s leading long-acting anti-diabetic drug, effective in reducing HbA1c, reducing weight in obese patients, and potentially effective in treating chronic diabetes kidney disease (DKD) and NASH. It is expected to be the first approved dual-targeted agonist for diabetes, obesity, and DKD in China.
- Developed with partner OPKO, LM008 is the most suitable anti-diabetic drug for treating diabetic patients in Asia, who has lower BMI on average, compared to diabetic patients in the West (US/Europe).
- Will bring more benefits for diabetes, obesity and DKD patients than all the existing GLP-1 drugs, based on its effect on weight and lipid reduction.
- A dual agonist of GLP-1R and Glucagon receptor. The unique mechanism and efficacy of LM008 has been demonstrated in over 800 patients globally. LM008 has been shown to reduce triglycerides and total cholesterol, improving the dyslipidemia, reducing body weight and visceral fat.
LM 007
- The world’s leading, third-generation and only long-acting FVIIa drug to treat hemophilia A&B and intraoperative hemorrhage. It will revolutionize the treatment of hemophilia, making acute treatment and long-term preventive treatment accessible to meet this urgent, unmet medical need.
- Exhibited a positive safety profile in both hemophilic patients and healthy subjects following a single IV or SC injection, respectively.
- Pharmacodynamics (PD) assessment of coagulation marker demonstrated a pharmacological activity of LM007 with an extended response.
Market
- There are over 1,000,000 hemophilia patients Asia and over 140,000 in China. (2 in every 100,000 Asians have hemophilia)
- Hemophilia is nationally declared as a critical rare disease in the ‘Rare-Disease Catalog’ in China.
- Many adolescents with hemophilia have life-long handicapped conditions with continuous need for critical care.
- There are no long-term effective and affordable treatments for hemophilia in China or Asia.
Development Plan
- Streamlined large-scale manufacturing ongoing in China
- IND preparation ongoing
- Accelerated approval pathway identified
- Preparation for Phase II/III in China ongoing
- LM007 is targeting initiation of Phase II/III trial for Hemophilia A & B by end of 1Q 2023, and approval in China by 2025.